Vaccine compliance
This guide explains how to view if a vaccine dose is compliant. You will likely meet vaccine requirements after retrieving your vaccine history in TrackMy Verivax (TMV). Most users only need to update their vaccination record once a year to show they received their annual Influenza dose.
Follow these steps to check your vaccine compliance.
If you have recently received a new vaccine dose, follow these instructions to update your vaccination record in TMV.
If you have not met the criteria for all vaccine requirements after updating your vaccination record in TMV, the following guide will help you understand what to do next.
View vaccine dose status
In Vaccines, click + to expand a vaccine record and view the compliance status and requirements.
The following are possible Status results and what they mean:
Pending: Your self-reported dose is awaiting review.
Dose Validated: Your self-reported dose is approved and contributes to your compliance.
Unvalidated or Failed Validation: Your self-reported dose is not approved.
Verified: These verified vaccine records are from TrackMy’s state registry search.
Next steps if a vaccine dose is marked as ‘Not Validated’
If your self-reported vaccine dose is not validated, your next step to correct your vaccination record will depend on the reason for the rejection.
Possible reasons for a self-reported vaccine dose to be rejected include:
Insufficient or incorrect documentation
You did not receive the vaccine within the required date range
The vaccine manufacturer is not accepted
The dose is a duplicate of a dose reported when you queried for your vaccine history in TMV
Below, the suggested next steps are explained for each scenario.
Your organization decides compliance requirements. Contact your organization if you do not understand the reason for the rejection.
If you cannot meet compliance with a dose, you may be able to request an accommodation.
Insufficient or incorrect documentation
To correct your documentation:
In your TMV portal, go to the Vaccines tile:

Expand the Vaccine type that the dose is for:
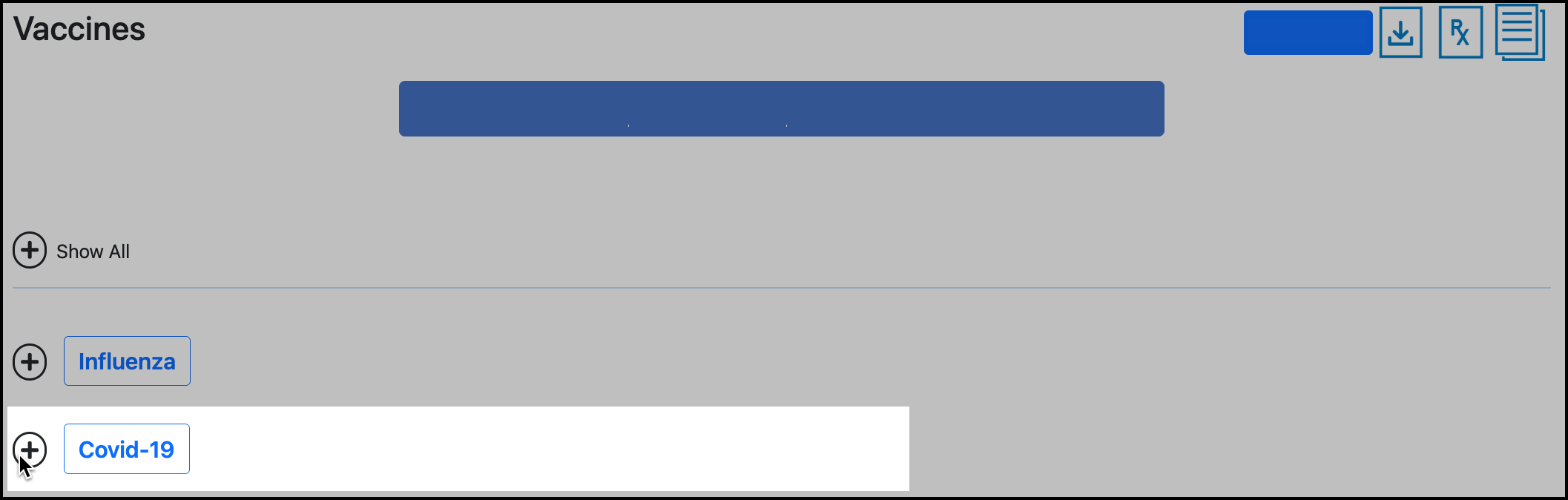
Hover over the ( ! ) icon to see the reason for rejection:
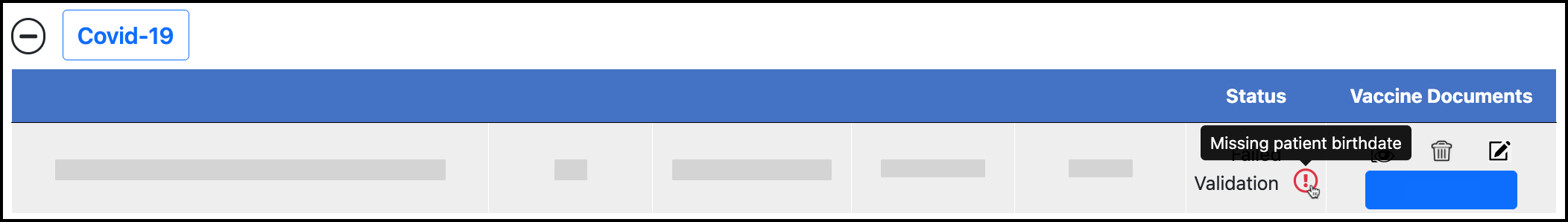
Click the Trash Can icon or Add Image for the dose:
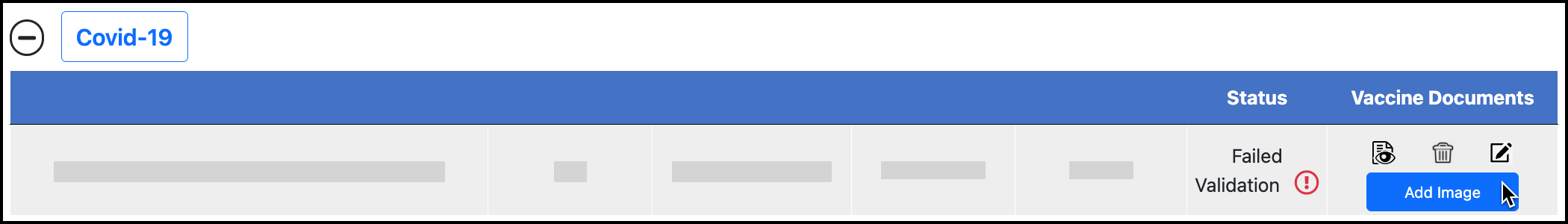
Upload the correct documentation.
After uploading the new documentation, your self-reported dose will return to the Pending status for review.
You did not receive the vaccine within the required date range
In this case, you may either need to receive a new dose of the vaccine to meet the date range requirement, or, correct your vaccine documentation to show you received the dose at the correct time.
For example, you are required to get the 2024 - 2025 Influenza dose. You self-reported an Influenza dose for 2024, but the uploaded documentation is for an Influenza dose you received in 2023.
There are two ways to correct this.
If you uploaded the wrong documentation by mistake:
Upload the correct documentation for the dose in TMV.
If you did not receive the 2024 - 2025 Influenza dose:
Receive the new Influenza dose and update your vaccination record in TMV. You may be able to request an accommodation or declination to excuse you from the dose requirement.
The vaccine manufacturer is not accepted
Ask your organization to confirm your next steps.
The dose is a duplicate of a dose reported when you queried for your vaccine history in TMV
No other steps are necessary because the correct dose is in TMV. Your self-reported dose was only rejected to avoid duplication.
