Self-report a vaccine dose
If you received a new vaccine dose and need to add this dose to TrackMy, run a new vaccination search in your state. Do not self-report a vaccine dose if you have not first searched for your record.
In some instances, TrackMy may not retrieve your complete vaccination record. You must self-report a vaccination if:
You received a vaccine dose outside the United States or in a U.S. territory.
You received a vaccine dose in a state TrackMy does not have an established health registry connection with.
TrackMy could not find a record of your vaccination based on the information provided.
Self-report a vaccine dose
In your TrackMy portal, click the Vaccines tile:

Click Add a Dose in the top-right corner:

Select the check box for the vaccine type(s) you are adding and click Continue.
Set the number of individual doses you are adding for each vaccine type and click Continue.
For each vaccine dose, enter your First Name, Last Name, Dose Date, and the state in which you received the vaccine dose.
If self-reporting a COVID-19 vaccination, enter the manufacturer. Review your immunization documentation carefully to report the correct vaccine type from your manufacturer.
Upload your immunization documentation. The uploaded document must display your name, date of birth, vaccine type and dose date for validation. Upload an image for each dose.
Select the check box to consent to sharing your vaccine dose with TrackMy Solutions and click Submit.
You can now view your self-reported vaccines in Vaccines. For a vaccine dose:
Click + to view the approval Status of a vaccine dose.
Click edit the details of a vaccine dose.
After you self-report your vaccination, TrackMy will review it. While your vaccine dose is awaiting review, your Status will be Pending. You may receive communication from TrackMy regarding your vaccine dose and any next steps.
The final status for the dose will show in your Vaccines dashboard.
The following are possible Status results and what they mean:
Pending: Your self-reported dose is awaiting review.
Dose Validated: Your self-reported dose is approved and contributes to your compliance.
Unvalidated or Failed Validation: Your self-reported dose is not approved.
Verified: These verified vaccine records are from TrackMy’s state registry search.
Contact your organization if you have any questions about vaccine requirements.
Managing your self-reported vaccine records
After submitting your self-reported vaccine dose, you can manage your self-reported documentation in your Vaccines dashboard.
To manage your self-reported vaccine documentation:
Click the eye icon to view your documentation:

Click Add Image to upload additional documentation:

Click the trash icon to delete and replace the current documentation:

Click the edit icon to edit the provided information for your vaccine dose, such as the administering state. This action is only available to self-reported vaccine doses with a status of Pending or Unvalidated:

Next steps if your dose is not approved
If your self-reported dose is not approved, your next step to correct your vaccination record will depend on the reason for the rejection.
Hover your mouse over the exclamation point (!) to view the reason for rejection:
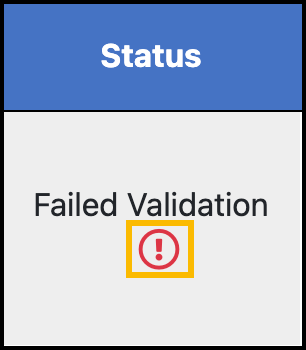
Your organization decides compliance requirements. Contact your organization if you do not understand the reason for the rejection.
Possible reasons for a vaccine dose to not meet compliance include:
Insufficient or incorrect documentation
You did not receive the vaccine within the required date range
The vaccine manufacturer is not accepted
If you cannot meet compliance, you may be able to file for an accommodation with your organization.
