Create vaccine survey and consent forms
If administering vaccines, you can manage the vaccine surveys and consent forms that patients complete on TrackMy.
Create a vaccine survey and consent form
In your TrackMy admin/clinical portal, click the Administration tile:

Go to Surveys and Consents and click Manage for the vaccine type.
Click Survey Builder.
Enter statements for consent checkboxes that will display on the form.
In Vaccine Information Documents, provide HTML links to vaccine information documents. Follow the following steps to create an HTML link to the documents:
Search for FDA information for the vaccine.
On the FDA’s page for the vaccine, click Fact Sheets.
Click the link for the correct fact sheet.
Copy and paste the URL of the document you are now viewing. Paste the URL and enter the name of the vaccine in the following template:
<a href="Your URL to fact sheet" target="_blank" class="d-inline-block mb-2 text-left mr-3">Name of the Vaccine the information is for</a>
Paste this html template completed with your information in Vaccine Information Documents.
To create a new question, click Add Question.
Enter the question and select the response type.
If the question is required, turn on the Required toggle.
Click Save.
New questions appear in Available Questions. Drag and drop a question to Current Questions to put the question on the questionnaire. You can drag and drop questions in your desired order.
Enter consent statement.
Turn on Enabled toggle to publish the vaccine survey and consent form. Turn off the toggle to save as a draft:
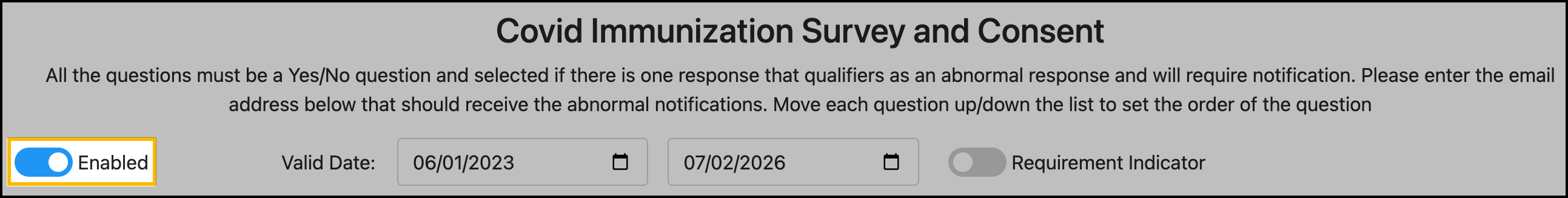
Click Save Survey and Consent.
